As a representative work of implant free concept, Drug coated balloon has received widespread attention since its launch. With the continuous improvement of research and development and technical process in recent years, coronary drug coated balloon products are also constantly being presented. While making technological progress, clinical research applications have also achieved considerable development. The new generation of drug-coated balloon FLOWTYTM Paclitaxel-Eluting PTCA Balloon Catheter adopts the latest technology. Through the unique patented technology and balloon surface modification technology, nano-scale paclitaxel is directly coated on the surface of the balloon. Without excipients, the occurrence of vascular inflammation and allergic reaction is avoided. At the same time, the nano-scale crystal particle size also ensures a high drug transfer rate during the expansion of the balloon and greatly reduces the risk of distal microvascular embolism during the operation. The results of FLOWTY clinical trial led by the team of Professor Wang Jian’an from the Second Hospital of Zhejiang University also fully confirmed its safety and effectiveness.
FLOWTYTM Paclitaxel-Eluting PTCA Balloon adopts randomized controlled, open label, multi-center clinical trial. After strict design and independent data analysis, the safety and reliability of its application in coronary stent restenosis are evaluated. A total of 211 patients were enrolled, including 105 cases in the experimental group (FLOWTYTM Paclitaxel-Eluting PTCA Balloon) and 106 cases in the control group (SeQuent Please Peripheral drug coated balloon catheter). The primary endpoint was target lesion in-segment late lumen loss at 9-month follow-up. The secondary endpoints were the target lesion in-segmental restenosis rate at 9 months postoperatively and the Patient-oriented cardiac outcome (POCE) at 12 months postoperatively.
In terms of the primary endpoint: the 9-month late lumen loss experimental group was 0.35±0.42mm, and the control group was 0.38±0.45mm, with a non-inferiority P value <0.001. It shows that compared with SeQuent Please, Flowty has reached the non-inferior endpoint.
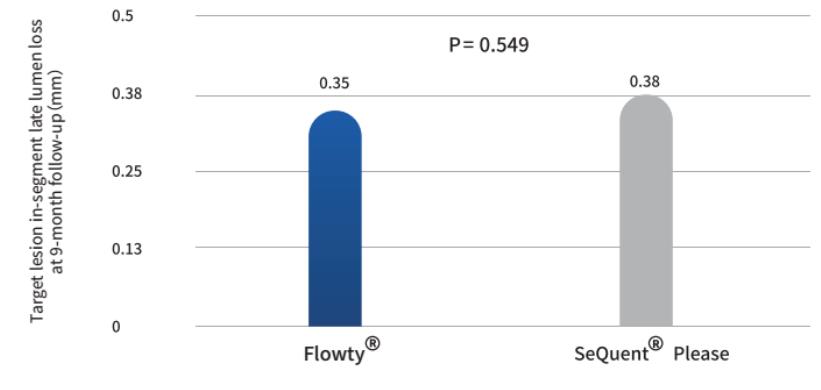
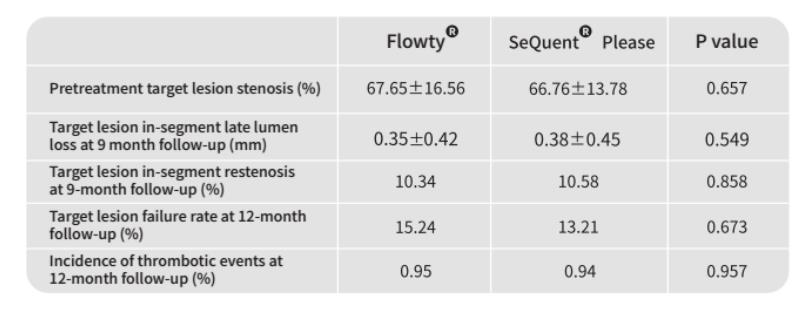
Clinical results of secondary endpoints are displayed in the table below. The results indicate that the safety and efficacy of Flowty are no different from SeQuent Please, which has the most sufficient clinical evidence.
To achieve such excellent clinical results, besides the serious scientific attitude and exquisite skills of the experts in each clinical center, it is also inseparable from the excellent technology of FLOWTYTM Paclitaxel-Eluting PTCA Balloon. As a new excipient-free drug-coated balloon, FLOWTYTM has very distinctive features:
✦ Patented excipient-free coating design, which improves the biocompatibility of the drug coating, reduces the inflammatory response of blood vessels, and avoids the damage caused by the excipients.
✦ Nano grade acicular paclitaxel directly acts on the blood vessel wall. Due to the high lipophilicity of paclitaxel and no interference from hydrophilic excipients, it can be quickly transferred to the blood vessel wall.
✦ The average particle size of the paclitaxel is less than 300 nanometers. It can greatly reduce the risk of distal microvascular embolism caused by the shedding of large particle coatings.
✦ The latest production and manufacturing process also ensures the uniformity and stability of the drug coating, ensuring sufficient drug delivery to the target lesion, reducing the drug loss rate and ensuring patient safety;
✦ The in vitro pharmacokinetic test shows that after the expansion of FLOWTYTM, the effective concentration in the tissue can be maintained for 28 days, which meets the clinical needs and can resist the occurrence of restenosis for a long time.
Implant-free is currently the most ideal treatment method for coronary interventional procedure, which can bring great long-term survival benefits to patients and retain more opportunities for patients’ follow-up treatment. At present, there are a variety of drug coated balloons in clinical application. FLOWTYTM Paclitaxel-Eluting PTCA Balloon is different from all other DCBs because of its unique excipient-free nano- acicular coating technology, which ensures high drug transfer while reducing the risk. Clinical trials have also shown that the brand-new FLOWTYTM Paclitaxel-Eluting PTCA Balloon is trustworthy in terms of safety and efficacy, which will ultimately benefit the patients.
